Mechanism for treating muscle degeneration discovered
Utrophin increase in muscle cells via transcriptional adaptation normalises cell function in Duchenne muscular dystrophy
Duchenne muscular dystrophy is a genetic disorder with severe consequences such as muscle atrophy. It is caused by mutations in the dystrophin gene - the longest gene in our genome - some of which result in disrupted gene expression. As a consequence, only mRNA fragments are formed during the process instead of intact mRNA chains. Researchers at the Max Planck Institute for Heart and Lung Research have now shown that thanks to these mRNA fragments, and via a process known as transcriptional adaptation, another similar protein, utrophin, is produced in larger quantities, thereby compensating for the lack of dystrophin. The scientists consider the study to be groundbreaking because it could pave the way for new therapeutic approaches for DMD as well as for other diseases.
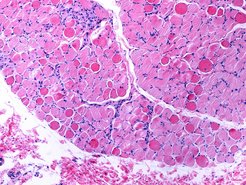
Duchenne muscular dystrophy (DMD) is a severe type of muscle disorder predominantly affecting boys. The disease is chronic and begins in childhood. The life expectancy of affected patients is significantly reduced. The disease, for which there is currently no cure, is caused by mutations in the dystrophin gene, which is located on the X chromosome. Dystrophin is important for cell membrane stability in muscle fibres. Due to the genetic defect, dystrophin is absent in these patients. As a consequence, muscle cells are restricted in their function and the musculature is increasingly weakened.
Treatment of Duchenne muscular dystrophy has so far been limited to maintaining muscle function for as long as possible using appropriate methods. In addition, initial gene therapies are being used to increase the production of dystrophin in muscle cells.
In a recent study, scientists from the Department of Developmental Genetics at the Max Planck Institute for Heart and Lung Research in Bad Nauheim headed by Didier Stainier have now gained crucial new insights into the genetic mechanisms underlying the development of DMD. The study could provide the basis for new therapeutic approaches.
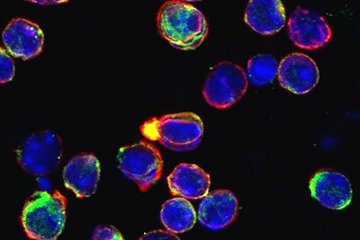
Besides dystrophin, the study focusses on a second protein called utrophin, which is related to dystrophin.
“It was already known that increased production of utrophin can at least partially compensate for the lack of dystrophin,” said Lara Falcucci, first author of the study. “We were now able to show for the first time in human muscle cells that at the level of gene expression, a process known as transcriptional adaptation is able to increase the production of utrophin.”
Until now, this was only known to occur in animal organisms, such as the nematode C. elegans, zebrafish, and mice.
“To understand the mechanism, it is important to know that in DMD, various mutations in the dystrophin gene prevent the production of a functional gene product, i.e. a protein. In addition, some dystrophin mutations cause the mRNA transcribed by the gene to break down into fragments. And these fragments surprisingly take on new functions in the regulation of other genes,” explains co-author Christopher Dooley.
The Max Planck scientists have now shown in cultured cells from DMD patients that the dystrophin mRNA fragments increase utrophin production in the affected cells. ‘The mechanism behind this observation is transcriptional adaptation. By regulating the dystrophin mRNA decay mechanism, it is possible to control utrophin production in these cells. This provides a starting point for a therapy,’ says Falcucci.
Didier Stainier, Director at the Max Planck Institute, puts the findings of the study into context: ‘Transcriptional adaptation is a fascinating process that allows us to mitigate the consequences of gene mutations. He therefore believes that his team have fundamentally improved understanding of the processes underlying genetic compensation and transcriptional adaptation in cells. ‘We are also convinced that this has opened the door to the development of new therapeutic approaches for the treatment of Duchenne muscular dystrophy. In particular, this could be the case for patients with mutations that have so far been difficult to address,’ says Stainier.